Multi-function Hand Push Model Weeder Tiller Machine Cultivators
Introduction
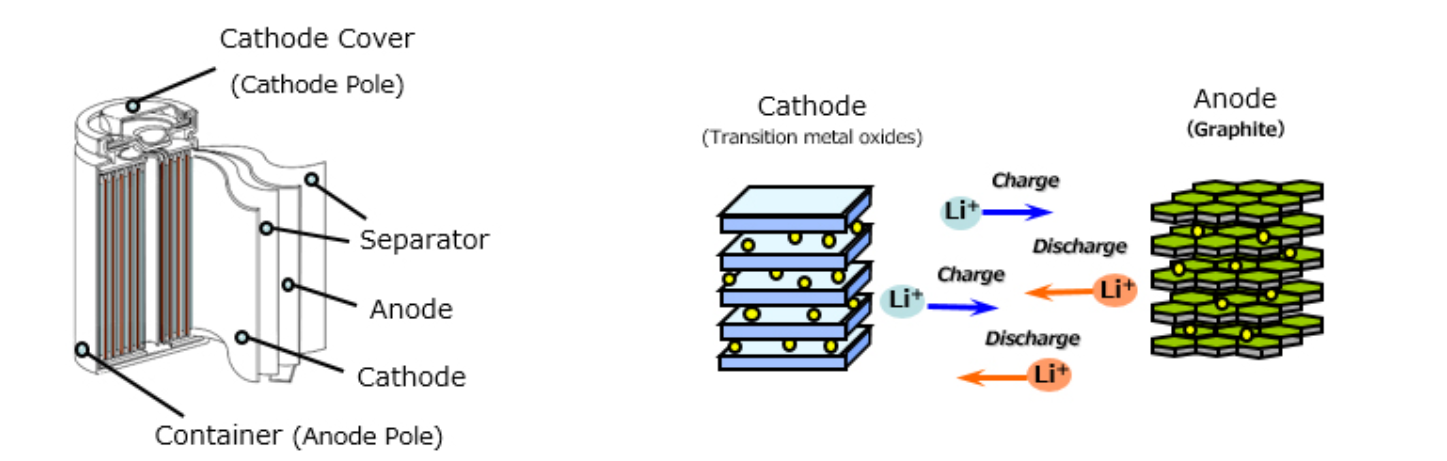
Lithium-ion batteries generate DC power by utilizing chemical reactions. When batteries are discharged and charged, lithium ions move back and forth between the electrodes (cathode and anode) inside of the batteries. In general, the cathode material is composed of Cobalt-base, Nickel-base or Manganese-base transition metal oxides and the anode material is composed of graphite.
Both cathode and anode are manufactured using a layered structure and the lithium ions are located in between layers. During charge, the lithium ions move from the cathode to the anode. During discharge, the lithium ions move from anode to the cathode.
High Capacity and High Safety are the strength of Panasonic batteries, especially High Capacity (or High Energy Density) batteries. As the capacity (or energy density) increases, it becomes more important to ensure the safety of batteries. As a result, Panasonic continues to develop better battery materials and manufacturing processes while also working to develop better battery control technology that will allow Panasonic batteries to be used safely, especially when layering up from cell to pack, module, and system. These activities help Panasonic’s batteries to maintain very high reliability.
By making the most of Panasonic batteries' strength, Panasonic can provide the best suitable battery solution for a wide variety of applications
Featured
High Energy Density: Lithium-ion batteries offer greater energy density versus other types of rechargeable batteries (Nickel Metal Hydride battery, Nickel-Cadmium battery, Lead Acid battery) which enables batteries to become smaller and lighter.
Great Power: As the operating voltage of Lithium-ion battery is higher versus other types of rechargeable batteries, it has the ability to support greater output.
Long Life: Rechargeable batteries can be used repeatedly by charging. The life of lithium-ion battery is defined as the number of full charge/discharge cycles. The greater the number of full charge/discharge cycles, the longer the batteries will last.








